Entropy and spontaneity :
`=>` Let us examine such a case in which `color{purple}(DeltaH = 0)` i.e., there is no change in enthalpy, but still the process is spontaneous.
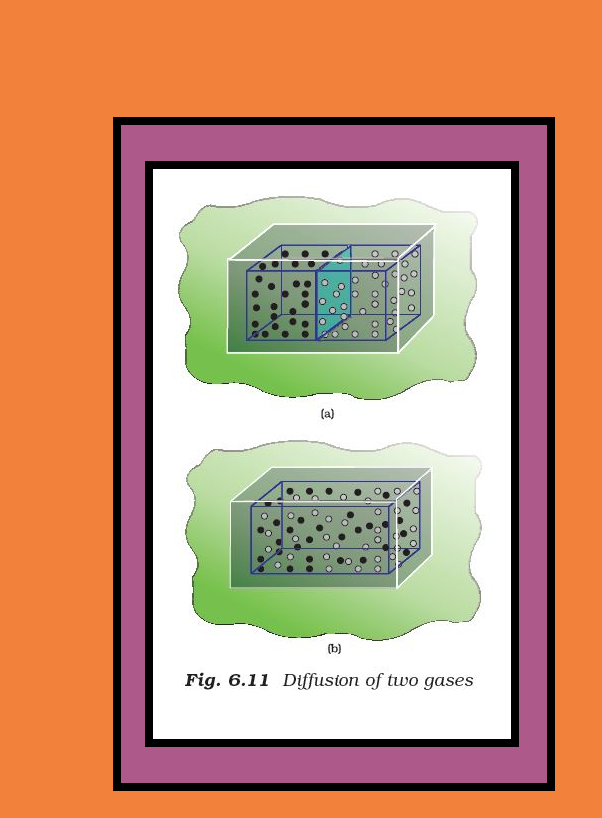
`=>` Let us consider diffusion of two gases into each other in a closed container which is isolated from the surroundings as shown in Fig.6.11.
● The two gases, say, gas `color{purple}(A)` and gas `color{purple}(B)` are represented by black dots and white dots respectively and separated by a movable partition [Fig. 6.11 (a)].
● When the partition is withdrawn [Fig.6.11( b)], the gases begin to diffuse into each other and after a period of time, diffusion will be complete.
`=>` Let us examine the process.
● Before partition, if we were to pick up the gas molecules from left container, we would be sure that these will be molecules of gas `A` and similarly if we were to pick up the gas molecules from right container, we would be sure that these will be molecules of gas `B`.
● But, if we were to pick up molecules from container when partition is removed, we are not sure whether the molecules picked are of gas `color{purple}(A)` or gas `color{purple}(B)`. We say that the system has become less predictable or more chaotic.
`=>` We may now formulate another postulate : In an isolated system, there is always a tendency for the systems’ energy to become more disordered or chaotic and this could be a criterion for spontaneous change.
`=>` At this point, we introduce another thermodynamic function, entropy denoted as `color{purple}(S)`.
● The above mentioned disorder is the manifestation of entropy.
`=>` To form a mental picture, one can think of entropy as a measure of the degree of randomness or disorder in the system.
`=>` The greater the disorder in an isolated system, the higher is the entropy.
`=>` As far as a chemical reaction is concerned, this entropy change can be attributed to rearrangement of atoms or ions from one pattern in the reactants to another (in the products).
● If the structure of the products is very much disordered than that of the reactants, there will be a resultant increase in entropy.
`=>` The change in entropy accompanying a chemical reaction may be estimated qualitatively by a consideration of the structures of the species taking part in the reaction.
● Decrease of regularity in structure would mean increase in entropy.
`=>` For a given substance, the crystalline solid state is the state of lowest entropy (most ordered). The gaseous state is state of highest entropy.
`=>` Now let's quantify entropy.
● One way to calculate the degree of disorder or chaotic distribution of energy among molecules would be through statistical method which is beyond the scope of this treatment.
● Other way would be to relate this process to the heat involved in a process which would make entropy a thermodynamic concept.
● Entropy, like any other thermodynamic property such as internal energy `color{purple}(U)` and enthalpy `color{purple}(H)` is a state function and `color{purple}(DeltaS)` is independent of path.
● Whenever heat is added to the system, it increases molecular motions causing increased randomness in the system. Thus heat (`q`) has randomising influence on the system.
● Can we then equate `color{purple}(DeltaS)` with `color{purple}(q)`? Experience suggests us that the distribution of heat also depends on the temperature at which heat is added to the system.
● A system at higher temperature has greater randomness in it than one at lower temperature. Thus, temperature is the measure of average chaotic motion of particles in the system.
● Heat added to a system at lower temperature causes greater randomness than when the same quantity of heat is added to it at higher temperature. This suggests that the entropy change is inversely proportional to the temperature.
● `color{purple}(DeltaS)` is related with `color{purple}(q)` and `color{purple}(T)` for a reversible reaction as :
`color{purple}(DeltaS = (q_(rev))/T)` .........(6.18)
● The total entropy change ( `color{purple}(DeltaS_text(total))`) for the system and surroundings of a spontaneous process is given by
`color{purple}(DeltaS_text(total) = DeltaS_text(system)+DeltaS_text(surr) > 0)` ...........(6.19)
● When a system is in equilibrium, the entropy is maximum, and the change in entropy, `color{purple}(DeltaS = 0)`
● We can say that entropy for a spontaneous process increases till it reaches maximum and at equilibrium the change in entropy is zero.
● Since entropy is a state property, we can calculate the change in entropy of a reversible process by
`color{purple}(DeltaS_{text(sys)} = q_{text(sys , rev}/T)`
● We find that both for reversible and irreversible expansion for an ideal gas, under isothermal conditions, `DeltaU = 0,` but `color{purple}(DeltaS_text(total)` i.e., `color{purple}(DeltaS_{text(sys)} +DeltaS_{text(surr)})` is not zero for irreversible process.
● Thus, `color{purple}(DeltaU)` does not discriminate between reversible and irreversible process, whereas `color{purple}(DeltaS)` does.
`=>` Let us consider diffusion of two gases into each other in a closed container which is isolated from the surroundings as shown in Fig.6.11.
● The two gases, say, gas `color{purple}(A)` and gas `color{purple}(B)` are represented by black dots and white dots respectively and separated by a movable partition [Fig. 6.11 (a)].
● When the partition is withdrawn [Fig.6.11( b)], the gases begin to diffuse into each other and after a period of time, diffusion will be complete.
`=>` Let us examine the process.
● Before partition, if we were to pick up the gas molecules from left container, we would be sure that these will be molecules of gas `A` and similarly if we were to pick up the gas molecules from right container, we would be sure that these will be molecules of gas `B`.
● But, if we were to pick up molecules from container when partition is removed, we are not sure whether the molecules picked are of gas `color{purple}(A)` or gas `color{purple}(B)`. We say that the system has become less predictable or more chaotic.
`=>` We may now formulate another postulate : In an isolated system, there is always a tendency for the systems’ energy to become more disordered or chaotic and this could be a criterion for spontaneous change.
`=>` At this point, we introduce another thermodynamic function, entropy denoted as `color{purple}(S)`.
● The above mentioned disorder is the manifestation of entropy.
`=>` To form a mental picture, one can think of entropy as a measure of the degree of randomness or disorder in the system.
`=>` The greater the disorder in an isolated system, the higher is the entropy.
`=>` As far as a chemical reaction is concerned, this entropy change can be attributed to rearrangement of atoms or ions from one pattern in the reactants to another (in the products).
● If the structure of the products is very much disordered than that of the reactants, there will be a resultant increase in entropy.
`=>` The change in entropy accompanying a chemical reaction may be estimated qualitatively by a consideration of the structures of the species taking part in the reaction.
● Decrease of regularity in structure would mean increase in entropy.
`=>` For a given substance, the crystalline solid state is the state of lowest entropy (most ordered). The gaseous state is state of highest entropy.
`=>` Now let's quantify entropy.
● One way to calculate the degree of disorder or chaotic distribution of energy among molecules would be through statistical method which is beyond the scope of this treatment.
● Other way would be to relate this process to the heat involved in a process which would make entropy a thermodynamic concept.
● Entropy, like any other thermodynamic property such as internal energy `color{purple}(U)` and enthalpy `color{purple}(H)` is a state function and `color{purple}(DeltaS)` is independent of path.
● Whenever heat is added to the system, it increases molecular motions causing increased randomness in the system. Thus heat (`q`) has randomising influence on the system.
● Can we then equate `color{purple}(DeltaS)` with `color{purple}(q)`? Experience suggests us that the distribution of heat also depends on the temperature at which heat is added to the system.
● A system at higher temperature has greater randomness in it than one at lower temperature. Thus, temperature is the measure of average chaotic motion of particles in the system.
● Heat added to a system at lower temperature causes greater randomness than when the same quantity of heat is added to it at higher temperature. This suggests that the entropy change is inversely proportional to the temperature.
● `color{purple}(DeltaS)` is related with `color{purple}(q)` and `color{purple}(T)` for a reversible reaction as :
`color{purple}(DeltaS = (q_(rev))/T)` .........(6.18)
● The total entropy change ( `color{purple}(DeltaS_text(total))`) for the system and surroundings of a spontaneous process is given by
`color{purple}(DeltaS_text(total) = DeltaS_text(system)+DeltaS_text(surr) > 0)` ...........(6.19)
● When a system is in equilibrium, the entropy is maximum, and the change in entropy, `color{purple}(DeltaS = 0)`
● We can say that entropy for a spontaneous process increases till it reaches maximum and at equilibrium the change in entropy is zero.
● Since entropy is a state property, we can calculate the change in entropy of a reversible process by
`color{purple}(DeltaS_{text(sys)} = q_{text(sys , rev}/T)`
● We find that both for reversible and irreversible expansion for an ideal gas, under isothermal conditions, `DeltaU = 0,` but `color{purple}(DeltaS_text(total)` i.e., `color{purple}(DeltaS_{text(sys)} +DeltaS_{text(surr)})` is not zero for irreversible process.
● Thus, `color{purple}(DeltaU)` does not discriminate between reversible and irreversible process, whereas `color{purple}(DeltaS)` does.



